
Steel is used everywhere in our daily lives, but we are not quite sure where it came from. Even though we know it is extracted from an iron ore, most of us may not know how an iron ore looks like, much less how it has been generated. This time, I am going to introduce one of its places of origin along with earth's history glimpsed by iron ores there.
Image - 1 Satellite image around Hemersley mining (ASTER/VNIR observed on 11th September, 2001)
For enlargement (jpeg file 2.5MB)The ASTER image is 18km x 18km with the mining at its center. ASTER band 3(near infrared), 2(red of visible light) and 1(green of visible light) are allocated to red, green, and blue of the three primary colors of light, respectively. Center of the image, which appears in green is the open pit mining area of iron ore. Iron ore (hematite, limonite) appears in green because it absorbs wavelength of band 1 and 3. Stripe pattern of the stratums is clear around the mine.
Image - 2 Satellite image of Hemersley area(ASTER/VNIR observed on 11th September 2001)
For enlargement (jpeg file 0.9MB)This is false color image of ASTER. You can see a question mark like form in the image, which appears in green and red. It is considered that huge amount of iron ores are deposited in the area. The image corresponds well to the geological map.
Figure - 1 Location of the image
I don't know how long it took for a train to cut across my way. It may have taken more than 10 minutes. In such a situation, it would be natural for people to be frustrated or get mad, but that wasn't the case with me. I was so much captured by the sight in a car.
I am not talking about a railroad crossing in Japan where trains come and go relentlessly nor that I am a train spotter. There was worth watching the sight.
I was on my way from Port Hedland in Australia toward inland when I had encountered a train carrying iron ores to port. There was no other car except mine. Numerous numbers of wagons were slowly dragged by only one locomotive engine.
The iron ores were extracted from a stratum generated 2.5 billion years ago at an open pit mining in Pilbara area. Iron ore layer can be viewed well from a valley in a national park located near the mine. People can go down the valley, which cuts in an arid plane deeply, to closely view iron ore walls.
The walls exhibit a red brown beautiful stripe pattern of stratum. The stripe is as small as a few millimeters when looked closely, but at the same time, one can see much bigger stripe patterns in distance. Stratum, which contains an iron ore is named banded iron formation after its stripe pattern.
The difference of the amount of iron contained in each layer contributes to form the stripe pattern. A layer containing larger amount of iron becomes an iron ore. Iron ore is the name of a rock, which consists mainly of oxide iron (magnetite, hematite, limonite and others). A layer containing less steel is made of rocks called chert, which mainly consists of quartz.
Hemersley in western Australia is the place where banded iron formation is extended as far as one can see. Open pit mining carried out in a mine there is so vast that it is possible to be pointed out from space. Trucks that carry out iron ore mined massively from the area are also gigantic, whose tires are taller than people.
Large scaled banded iron formation was mostly formed approximately 2.5 billion years ago, and all the iron ore there had been formed no later than 1.9 billion years ago.
What can be said by the fact that most of the banded iron formations occurred coincidentally and stopped all of a sudden? Since a large scaled banded iron formation exists all over the world, it is estimable that some kind of worldwide phenomenon has occurred back in those days.
Banded iron formation is a kind of sedimentary rock. Since sedimentary rock occurs at the bottom of seas, iron ores occur when iron deposits at the sea bottom. Usually, iron is dissolved in seawater in the form of ion, Fe2+. However, if oxidation progresses due to some reasons to make it Fe3+, chemical reaction occurs to make it Fe(OH)3, and it deposits. Due to dehydration, deposited iron hydroxide alters into iron oxide in a long time.
Existence of banded iron formations indicates that iron ions dissolved in seawater were oxidized in the wake of a certain event occurred on a global scale. It is considered that creatures, which generate oxygen, have come to exist massively in seawater when the formation occurred. The creature, which generated oxygen is photosynthetic organism.
A scenario for banded iron formation is like this:
Photosynthetic organism (cyanobacteria), which came to exist more than 3 billion years ago, irrupted 2.5 billion years ago. Iron ion which had been dissolved in seawater until then, started to deposit by oxidation in the wake of a sudden increase in the number of photosynthetic organism. Iron precipitated under water while photosynthetic was active in certain seasons or times of a day, whereas ordinary sediments (chert) deposited while photosynthetic was inactive. Constant change in biological activities like this have played a role in forming the stripe pattern of iron ore.
When most of the iron ions dissolved in water had deposited, the amount of iron ion and oxygen struck a balance (equalized). When oxygen outnumbered iron ion in seawater, it started to rise above seawater to comprise an essential component of the atmosphere.
Large scaled banded iron formations existing worldwide are the proof that oxygen had come to exist on earth in a limited period of time. An event of organism irruption brought about the sudden formation of oxygen, contributing to the concentration of iron resources underwater around 2.5 billion years ago. Otherwise, it might have been impossible for human beings to extract iron as a material as we do today.
Since modern civilization of the world today is built up on iron structures, it would have been quite a difference if iron resource had been slowly formed, which makes it difficult to extract. In that case, it might have been in the Stone Age or Bronze Age by now, yet to tell the arrival of Iron Age.
1st April, 2003
Yoshiyuki Koide
 |
Picture - 1 A long train carrying iron ores Countless wagons are dragged by one locomotive engine. It takes time all the more for the train to go through a railroad crossing because it runs slowly. Iron ores are being carried from the mine to a nearby port regularly. Iron ores there are also exported to Japan. |
 |
Picture - 2 The open pit mining of iron ore The open pit mining far exceeded my expectation in terms of scale and area. Iron ores mined in the area are transported to all over the world for use as a resource. Rocks that iron cannot be extracted are buried back to fill up the ground again and environment-friendly mining methods are applied to the site. |
 |
 |
|
|
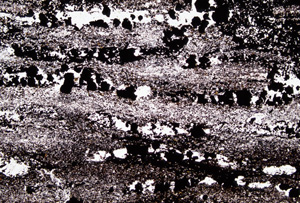 |
Picture - 5 Micrograph of banded iron formation You can find a stripe pattern even in a microscopic view. In the picture, there are multiple of layers with many black granules of iron oxides and chert layers, which consists of white or transparent granules.
|
Copyright of Image - 1 and 2 belongs to JSS and text and Picture - 1 through 5 belongs to Prof. Yoshiyuki Koide of Sapporo Gakuin University. Permission of JSS is needed to their use for other purposes.